Factors associated with post-stroke fatigue
When nurses and healthcare personnel are able to identify which patients are particularly at risk of post-stroke fatigue, patients can be given the appropriate follow-up at the right time.
Background: Fatigue is a pronounced symptom that often affects stroke patients. Fatigue is described as persistent tiredness, exhaustion and reduced capacity for activity, and is often associated with impaired quality of life. Earlier studies show a large variation in associated factors and the prevalence of post-stroke fatigue (PSF).
Objective: To investigate whether there is an association between PSF and socio-demographic, medical and clinical characteristics in a Norwegian sample.
Method: A cross-sectional study of 321 stroke patients recruited from 11 hospitals in Norway in the period 2014–2016. We collected data using a structured questionnaire in individual interviews held 4–6 weeks after the patient’s stroke. Fatigue was measured using a shortened version of the Fatigue Questionnaire (FQ). We used multivariate logistic regression analysis in this study.
Results: The prevalence of PSF was 43.6 per cent in this study. Previous health problems (OR, odds ratio = 4.99), depression (OR = 4.47), care responsibilities for others (OR = 2.86) and the severity of the stroke (OR = 1.16) were factors that increased the risk of fatigue 4–6 weeks after a stroke.
Conclusion: The study supports earlier findings that PSF is widespread. The study identified four factors that increased the likelihood of reporting fatigue 4–6 weeks after a stroke. Knowledge of the prevalence of PSF and the factors associated with PSF is crucial for nurses and other healthcare personnel being able to identify which patients may need follow-up in connection with PSF.
Every year, approximately 15 000 people have a stroke in Norway (1). Fatigue often occurs as a subjectively experienced state following a stroke accompanied by a substantially reduced capacity for activity, known as post-stroke fatigue (PSF) (2, 3). Around 10 per cent of the Norwegian population suffer from exhaustion or fatigue (4), but the prevalence of PSF is higher (3).
A stroke is the most common cause of disability in the elderly, but appropriate and early rehabilitation increases the likelihood of regaining everyday functions (1). Many patients experience considerable progress as a result of targeted rehabilitation, but studies show that PSF can last for several years (5, 6). Studies have also shown that PSF can occur at different points in time following a stroke (7).
PSF is associated with impaired quality of life, a reduced degree of coping (8), a reduced capacity to perform daily tasks (9) and loss of control (10).
What is fatigue?
Schillinger and Becker (3) suggest that fatigue can be defined as ‘a subjective experience of protracted or recurrent tiredness and a reduced capacity for mental and/or physical activity’. PSF is a state that is characterised by exhaustion and a considerable depletion of energy that often occurs without prior physical or mental exertion (3).
The cause of PSF is unknown, but there is likely to be a complex causal explanation (11). It is not clear what medical and socio-demographic factors are associated with PSF as findings in earlier studies are contradictory (3, 8, 12, 13). However, PSF is often associated with pain and sleep disorders (8) and is closely associated with depression (8, 9, 14) and anxiety (8, 10, 14).
It is uncertain whether socio-demographic factors such as age, gender, marital status, living situation (living alone or not), education and returning to paid work are associated with PSF (3, 8). Previous research findings are also contradictory in terms of the association between PSF and neuropsychological factors, such as the stroke location, type and severity (3, 8).
How is fatigue measured?
The prevalence of PSF shown in earlier studies varies. Three systematic review articles covering 71, 16 and 22 studies respectively show a prevalence of 51–72 per cent (3), 29–70 per cent (12) and 25–35 per cent (13) for PSF. The review articles point out that different definitions of PSF are used and that prevalence measurements vary depending on which operationalisation of PSF is used.
There are several measuring instruments for measuring fatigue (15), but none specifically for PSF. Fatigue is a subjective experience that can only be reported by the patient, and instruments for self-reporting are therefore used. Different scales are often used to measure PSF, but there is no set threshold for fatigue (3), which can complicate the interpretation of data from this type of measuring instrument.
Objective of the study
The cause of PSF is unclear, treatment is limited and figures on the prevalence of PSF vary considerably (3). Earlier research has identified a clear need for more research in order to secure the best possible follow-up for patients (3, 8, 16). Knowledge of how many and which patients are struggling with PSF is therefore needed so that these patients can receive targeted treatment.
The objective of this study is to investigate whether there is an association between PSF and socio-demographic, medical and clinical characteristics in a Norwegian sample.
Method
Design and sample
This study is a cross-sectional study using randomised controlled trial (RCT) data to investigate the effect of a psychosocial intervention on stroke patients (17). The sample in the cross-sectional study consists of stroke patients recruited from 11 hospitals in Southeast Norway during the period November 2014 to November 2016.
A total of 353 patients met the inclusion criteria and consented to taking part in the study (Figure 1). Thirty-one participants dropped out between the recruitment phase and the data collection, and one participant failed to answer questions about fatigue. A total of 321 participants were therefore included in our cross-sectional study.
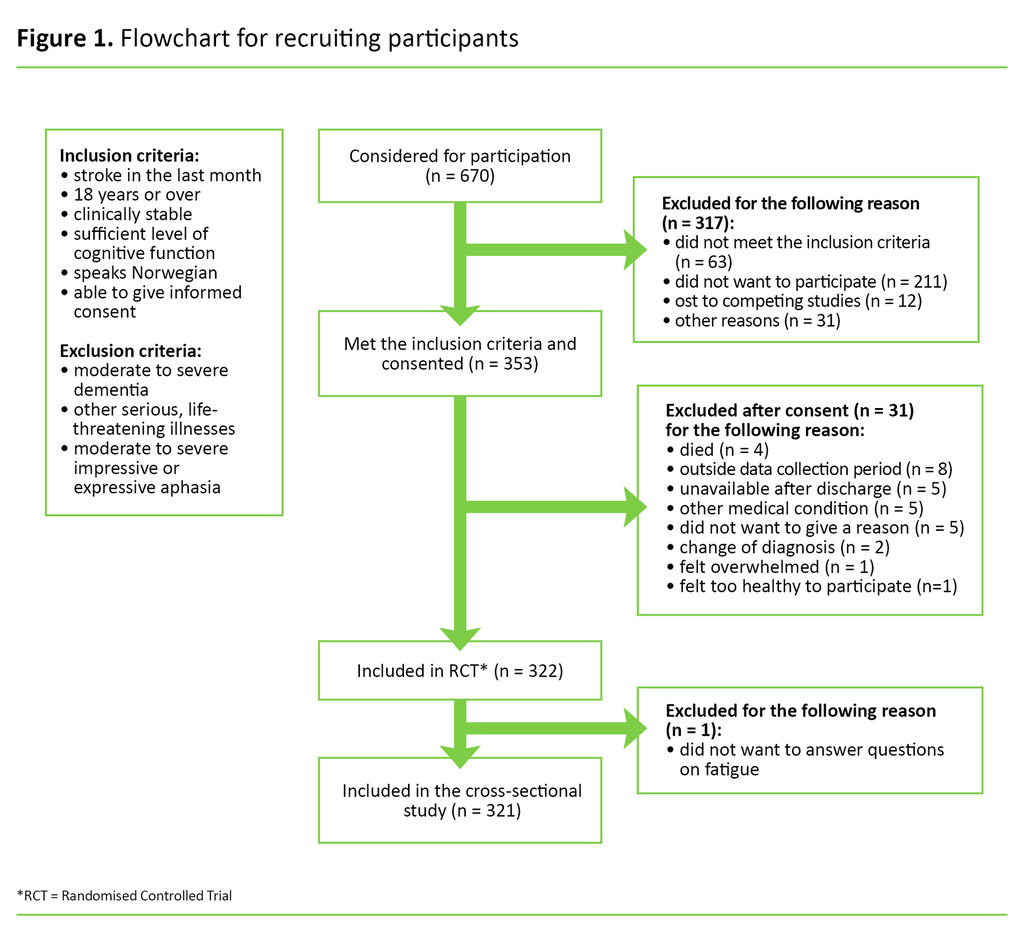
Data collection and instruments
We collected the data 4–6 weeks after the patient’s stroke, using a structured questionnaire in individual interviews. The questionnaire was made up of six measuring instruments (17). In this cross-sectional study we have only used one of these instruments to measure fatigue and one to measure depression. The data were collected by nurses and ergonomists who are trained in using the measuring instruments.
In addition to data on fatigue and depression, we also collected data on socio-demographic characteristics, such as age, gender, marital status, living situation, education, care responsibilities, pre- and post-stroke work situation, as well as medical and clinical data on the severity, type and location of the stroke, rehabilitation services and health problems prior to the stroke.
We measured the prevalence of fatigue and operationalised PSF using a shortened version of the Fatigue Questionnaire (FQ), which consists of one closed question: ‘Do you often feel tired, indisposed and lacking energy?’. Patients who answered in the affirmative were asked a follow-up question about the duration of the symptoms, with the response options ‘less than 1 week’, ‘less than 1 month’, ‘1–3 months’, ‘3–6 months’ and ‘6 months or more’ (4, 18).
The keywords ‘often’, ‘tired’, ‘indisposed’ and ‘lacking energy’ and the reported duration of these symptoms relate to the definition of fatigue as a protracted and recurrent experience of lacking energy and a reduced capacity for activity compared to before the stroke (3).
We measured depression using the Yale-Brown single item screening questionnaire (Yale), which consists of one question: ‘Do you often feel sad or depressed?’. The response options were ‘yes’ and ‘no’ (19). Yale is an instrument for screening depression that is validated for stroke patients. The instrument is considered to have satisfactory sensitivity and specificity compared to more comprehensive instruments for identifying depression (19).
In the acute phase at the hospital, medical staff assessed the severity of the stroke according to the National Institutes of Health Stroke Scale (NIHSS). NIHSS consists of 11 clinical observations that are quantified and tallied up on a scale from 0–42. The higher the score, the higher the degree of disability (20).
Analysis and coding
We used descriptive statistics to describe the sample in this study. Multivariate logistic regression analysis was used with PSF (0 = ‘no, no reported fatigue’, 1 = ‘yes, recently occurring fatigue’) as the dependent variable. PSF was operationalised with ‘1 = yes’ on the variable FQ if the fatigue symptoms occurred recently (<3 months’ duration). Participants who reported fatigue symptoms for a longer duration (>3 months) (n = 35) were excluded from the logistic regression analysis.
Based on earlier research into factors that may be associated with PSF (3, 8, 12, 13, 21), we included the following variables as independent variables in the logistic regression: age at admission, gender (1 = male), care responsibilities for others (1 = yes), previous health problems (1 = yes), stroke type (0 = infarction, 1 = cerebral haemorrhage), severity of stroke (total score on NIHSS) and depression (1 = yes).
The post-stroke work situation (0 = in work, 1 = retired/receiving welfare benefits/ unemployed, 2 = on sick leave) was included in the model in order to check for potential confounding. The sample for the logistic regression analysis only includes patients with complete medical and clinical data (n = 209). We set the level of significance in all the statistical analyses to 0.05. Statistical analyses were performed in SPSS version 25.
Ethical considerations
The study was approved by the Regional Committees for Medical and Health Research Ethics (REC number 2013/2047) and registered with the Data Protection Officer (registration number 2014/1026) for the health trusts that were involved in the recruitment process in the study. The participants gave voluntary consent to taking part in the study and were free to withdraw at any time without giving a reason or facing consequences in respect of further medical follow-up.
Results
Description of the sample
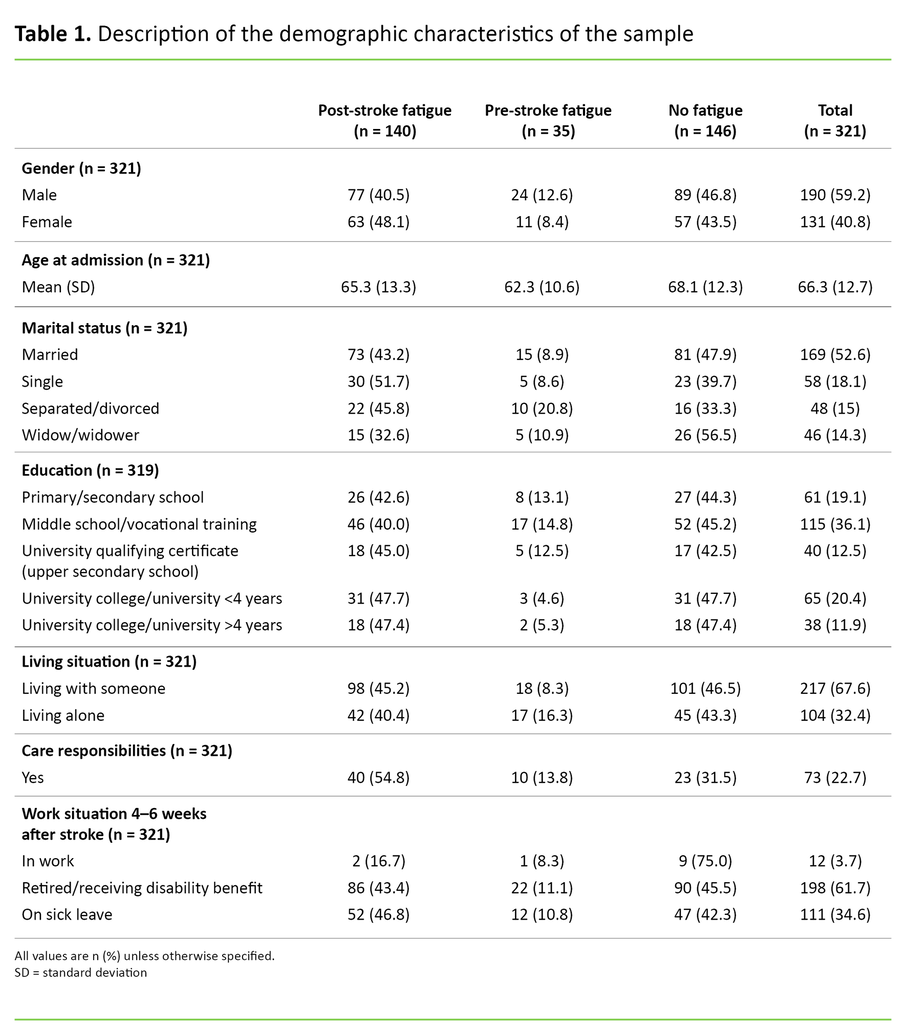
The sample in this study was made up of 59.2 per cent men and 40.8 per cent women, with a total mean age of 66.3 years. The proportion who had care responsibilities for their own children or someone else close to them was 22.7 per cent (Table 1).
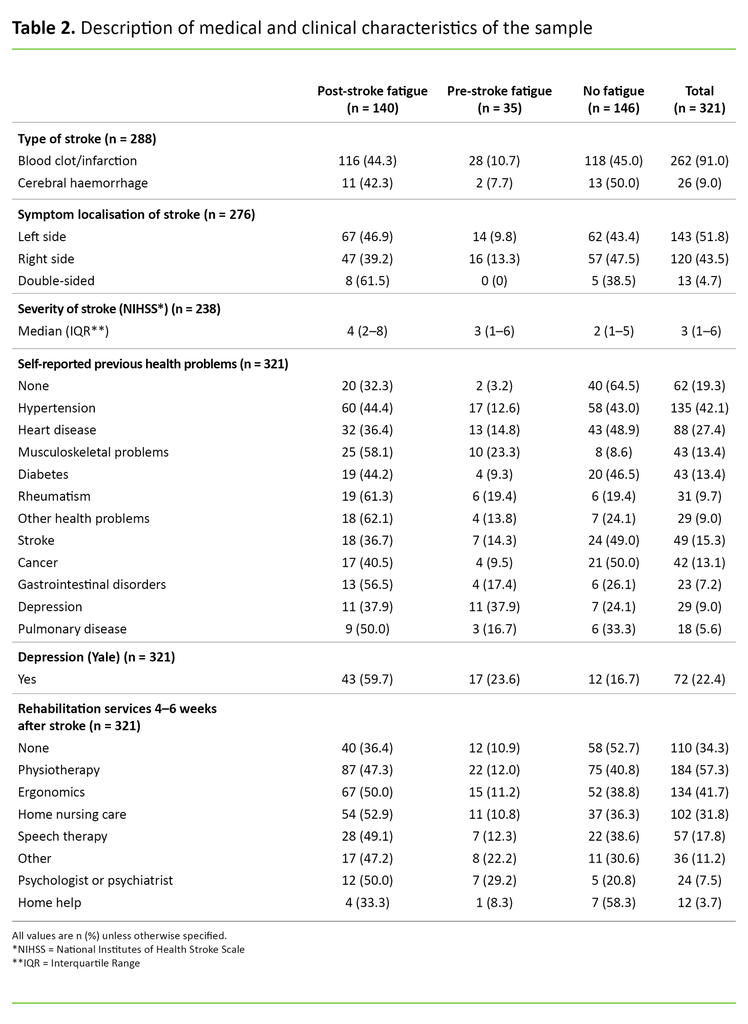
In 91 per cent of cases, blood clotting was the cause of the stroke, while 9 per cent were due to a cerebral haemorrhage. Overall, the median score for the stroke severity was NIHSS 3, and the interquartile range was 1–6. Only 19.3 per cent reported no previous health problems. A total of 22.4 per cent reported depression 4–6 weeks after the stroke. Participants reported that they made extensive use of rehabilitation services 4–6 weeks after the stroke. Only 34.3 per cent reported that they did not receive any form of rehabilitation (Table 2).
Prevalence and factors associated with PSF
The prevalence of PSF was 43.6 per cent. Of those who reported PSF, 55 per cent were men and 45 per cent were women, with a mean age of 65.3 years. A total of 10.9 per cent of the participants reported pre-stroke fatigue, i.e. fatigue before the stroke, while 45.5 per cent did not experience fatigue 4–6 weeks after the stroke.
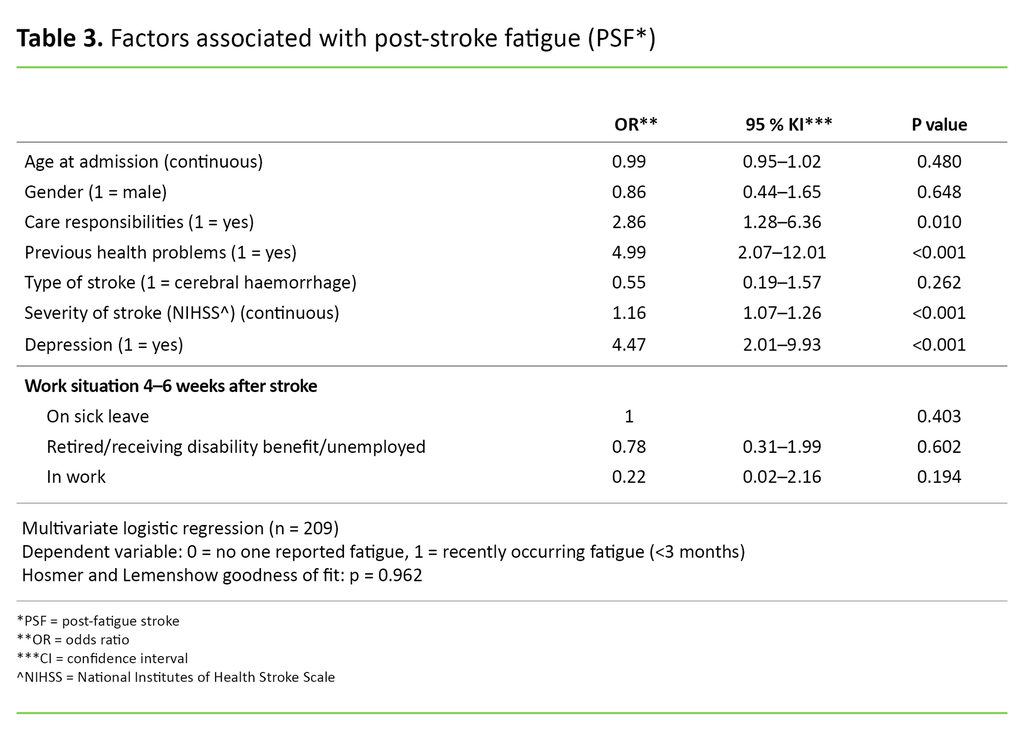
The logistic regression analysis showed that four factors were associated with PSF when we controlled for the other factors in the model (Table 3). Patients who reported having health problems before the stroke were nearly five times more likely (OR = 4.99; 95% CI 2.07–12.01; p <0.001) to report PSF compared to patients who did not report previous health problems.
Patients who reported depression 4–6 weeks after their stroke were more than four times more likely (OR = 4.47; 95% CI 2.01–9.93; p <0.001) to report PSF compared to patients who did not report depression. Those with care responsibilities for others were more than 2.5 times more likely (OR = 2.86; 95% CI 1.28-6.36; p = 0.01) to report PSF compared to those who did not have care responsibilities.
For each point in the NIHSS score, the patient was 1.16 times more likely to report PSF (OR = 1.16; 95% CI 1.07-1.26; p <0.001). The patient’s gender, age, work situation and type of stroke had no statistically significant association with PSF in this model.
Discussion
Prevalence of PSF
A total of 43.6 per cent of participants reported PSF 4–6 weeks after their stroke. This figure concurs with the prevalence of PSF shown in previous studies (12, 13). Studies show that PSF may increase over time. One study found that the prevalence of PSF increased from the date of admission to three months after the stroke (7). Another study showed that the prevalence at admission, and six and twelve months after the stroke was 52, 64 and 70 per cent respectively (22).
The measurement period of 4–6 weeks after the stroke may explain the medium to low prevalence in our study compared to previous studies, but it may also mean that previous studies used other criteria for PSF.
The definition of PSF is not standardised in the research literature, and there are no specific measuring instruments for PSF (3). The large variance in the prevalence of PSF from earlier studies (3) may be because the term ‘post-stroke fatigue’ is defined and operationalised in different ways.
Fatigue is often described as tiredness or lack of energy, which is associated with biological, psychological and social factors (11). Earlier studies hypothesise that the term PSF should be defined more uniformly, for example, by distinguishing between physical and mental fatigue (23). The prevalence of PSF appears to be somewhat lower in, for example, Asia (35 per cent) than in Europe (13), which indicates that social factors may be linked to the experience of fatigue.
Other pre-stroke health problems increased the risk of PSF
In this study, we found that patients who reported other pre-stroke health problems were almost five times more likely to report PSF. There is currently insufficient research on the types of health problems that are predictors of PSF, but both depression and pre-stroke fatigue are factors that have been shown to have an association with PSF (24, 25). Researchers have been aware of a possible association between premorbid factors and PSF for some time.
A study conducted in 2005 found that pre-stroke fatigue was the factor that made it most likely to experience PSF, but that a higher stroke severity and post-stroke depression were also among the most important factors for experiencing PSF (24). The association of both a higher stroke severity and post-stroke depression with PSF concurs with our findings.
Pre-stroke fatigue may be an important factor for PSF, but our study does not show a significant finding that it is associated with PSF as we did not measure pre-stroke fatigue as a separate factor.
Higher severity of stroke increased the risk of PSF
Earlier research suggests little association between stroke severity and PSF (3). Findings from our study showed that stroke severity was a significant factor that increased the risk of PSF, with each point in the NIHSS score increasing the risk of PSF by 1.16. However, several previous studies do not show to any extent that medical and clinical factors are important for experiencing PSF (3, 8).
A Norwegian systematic literature review concluded that stroke severity has either no association or a questionable association with PSF, since none of the studies in the review found any significant association between the severity of a stroke and PSF (3). However, one study found that stroke severity proved to be a factor that had a significant association with PSF (24).
These contradictory findings may be due to differences in study design, the operationalisation of fatigue or the sample size. The findings in our study indicate that the higher severity of the stroke may be significant for PSF. The type of stroke has an uncertain association with PSF (3), and we also found no association between these factors in our study.
Depression closely associated with PSF
The results of this study showed a strong association between fatigue and depression 4–6 weeks after the patient’s stroke. The likelihood of reporting PSF was more than four times greater if depression was reported at the same time. This finding is consistent with earlier research, which has shown that depression is widespread among those who experience PSF (14, 26, 27).
A total of 22.4 per cent reported depression. The proportion who reported PSF at the same time was 59.7 per cent, and the prevalence was only 16.7 per cent among those who did not report PSF. There is no doubt a complex explanation as to why depression occurred so often with fatigue. Fatigue and depression are probably different parts of the same spectrum with similar causal explanations (11).
Lack of energy is often included as a criterion in different definitions and measurements of depression. It is therefore possible that the instruments measure different parts of a phenomenon in the same domain. Part of the covariance may be a reflection of linguistic challenges in the measuring instruments. Fatigue is characterised by a reduced capacity to perform daily tasks, which in turn can lead to depression and impaired quality of life (9).
Patient’s concerns about their prospects can also impact on fatigue, and some patients may be more at risk of PSF due to health problems prior to the stroke. Depressive symptoms and pre-stroke fatigue are both factors that have been found to have a possible correlation with PSF (25).
It is interesting to compare the associations between the patient’s health before the stroke and PSF with the findings of this study. The findings showed that the prevalence of post-stroke depression was higher among those who also reported experiencing fatigue before the stroke, compared to those who did not report PSF. However, the prevalence of post-stroke depression was highest among those who reported experiencing fatigue more recently after the stroke (Table 2). There is an unclear distinction between the three phenomena of fatigue, depression and anxiety after a stroke (14).
Associations between life situation and PSF
PSF may have an important association with psychosocial factors. In our study, we found that having care responsibilities for others was a significant factor that increased the risk of PSF. One explanation for the association between care responsibilities and PSF is the stress levels involved in an acutely ill patient caring for him/herself whilst also caring for others, such as children, a spouse or someone else close to them.
This association indicates that PSF should be viewed in the context of the stroke patient’s life situation (21). The work situation was shown to have no effect on whether patients in our sample experienced PSF.
The impact of age on PSF has previously been studied, but the association is unclear (3). One study found that older people experience PSF more often than young people (22), and it has also been found that the prevalence is higher among younger stroke patients (28). Our findings show no association with age.
The fact that pre-stroke health problems and stroke severity increased the risk of PSF in this study suggests that biological factors may play a role in increasing the likelihood of experiencing PSF. A social factor such as having care responsibilities for others and experiencing post-stroke depression increased the risk of PSF. This shows that PSF can be a complex phenomenon. It is unclear whether PSF primarily has a medical causal explanation, or whether there are psychosocial and environmental factors that increase the likelihood of experiencing PSF (11).
Strengths and weaknesses of the study
Operationalisation of fatigue
The instrument and the question formulation we have chosen to operationalise fatigue serve as a guide for how PSF is interpreted in this study. Selecting one response option in structured questionnaires can be difficult, and this may have led to over or underreporting. The question formulation may, in turn, affect whether PSF is measured correctly in terms of how the phenomenon actually affects the patient.
The way we defined the duration of the fatigue symptoms may be a weakness of the study, as the response options given to the patient do not define ‘before’ or ‘after’ the stroke very precisely. We could have achieved greater precision if there had been more response options for the questions concerning duration.
Defining and operationalising the term PSF can be a challenge in that there is no validated instrument for measuring the phenomenon of PSF, i.e. fatigue after a stroke. PSF can manifest itself in different ways in contexts other than after a stroke. In our study, it was appropriate to distinguish between the patients who unambiguously reported protracted fatigue (>3 months), as we wanted to investigate fatigue that occurred after a stroke. We cannot rule out the possibility that those who reported fatigue of a longer duration interpreted the question about fatigue in the same way as those who experienced recently occurring fatigue. Nor can we ignore the possibility that the results would have been different if we had used other instruments.
Although there is still uncertainty about the best way to measure PSF, this study generated new knowledge about which patients experienced fatigue 4–6 weeks after a stroke, and may therefore help to identify which patients may be at risk of impaired quality of life after a stroke (6, 9, 10).
The span of the reported confidence intervals (95% CI) from the logistic regression analysis (Table 3) is relatively broad. This is probably due to the fact that a small sample gives greater variation around the true estimate. This means that even if the results are statistically significant, the estimated odds ratio for each of the variables should be interpreted with caution.
Representativeness of the sample
Following the recruitment protocol at each hospital presented a number of challenges. This may have meant that some patients who met the inclusion criteria were not asked to participate, which in turn may have led to unintentional bias in the sample. Some patients did not want to participate in the study, partly because they were too ill. The patients who had more severe outcomes and more pronounced fatigue may have chosen not to participate in the study because they thought it was too demanding.
Another reason why patients did not want to participate in this study was that they felt too healthy. When patients feel too ill to participate in a study, it can lead to bias in the sample, or mean that only the healthiest patients are represented in the study. Compared to the population registered in the stroke register (29), the participants in our study are 10 years younger on average. The proportion of women is 5 per cent lower, and the proportion who had a minor stroke (NIHSS <6) is 5 per cent higher.
Data collection by means of a structured questionnaire in individual interviews can lower the patient’s threshold for completing the questionnaire, resulting in a higher completion rate. This type of approach helps to gather more complete data and prevents data omissions. It also reduces the possibility of misunderstanding the questionnaire.
One of the strengths of this study is that it uses data from a multi-centre study that has recruited patients from several different hospitals in the country. Furthermore, we include a large number of participants in the study. These elements increase the generalisability of the results to other stroke patients.
Conclusion
In our study, the prevalence of fatigue in patients 4–6 weeks after a stroke was 43.6 per cent. The following factors increased the risk of PSF in the sample: health problems before the stroke, depression after the stroke, care responsibilities for someone close and higher severity of stroke (measured by NIHSS).
Nurses and healthcare personnel need to know who is particularly at risk of PSF so that these patients can be given the appropriate follow-up at the right time. It is therefore important to know the prevalence of PSF and the factors associated with PSF. However, more research is needed on PSF.
Thank you to Manuela Zucknick, a statistician in the Department of Biostatistics, University of Oslo, for helping with the quality assurance of statistical analyses and interpretations.
Our study was funded with support from South-Eastern Norway Regional Health Authority (project number 2013086) and the European Commission’s seventh framework programme (FP7-PEOPLE-2013-COFUND) (agreement number 609020 – Scientia Fellows). The University of Oslo and Oslo University Hospital, Ullevål, have contributed research time and administrative support to the study.
References
1. Ellekjær H, Selmer R. Hjerneslag – like mange rammes, men prognosen er bedre. Tidsskr Nor Legeforen. 2007;127:740–3.
2. Kirkevold M, Christensen D, Andersen G, Johansen S, Harder I. Fatigue after stroke: manifestations and strategies. Disabil Rehabil. 2012;34(8):665–70.
3. Schillinger A, Becker F. Fatigue/utmattelse etter traumatisk hjerneskade og hjerneslag. Tidsskr Nor Legeforen. 2015;135:331–5.
4. Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res. 1998;45(1):53–65.
5. Elf M, Eriksson G, Johansson S, von Koch L, Ytterberg C. Self-reported fatigue and associated factors six years after stroke. PLoS One. 2016;11(8):e0161942.
6. Lerdal A, Gay CL. Fatigue in the acute phase after first stroke predicts poorer physical health 18 months later. Neurology. 2013;81(18):1581–7.
7. Delva II, Lytvynenko NV, Delva MY. Post-stroke fatigue and its dimensions within first 3 months after stroke. Wiad Lek. 2017;70(1):43–6.
8. Ponchel A, Bombois S, Bordet R, Hénon H. Factors associated with poststroke fatigue: a systematic review. Stroke Res Treat. 2015;2015:347920.
9. Mandliya A, Das A, Unnikrishnan JP, Amal MG, Sarma PS, Sylaja PN. Post-stroke fatigue is an independent predictor of post-stroke disability and burden of care: a path analysis study. Top Stroke Rehabil. 2016;23(1):1–7.
10. Wu S, Barugh A, Macleod M, Mead G. Psychological associations of poststroke fatigue: a systematic review and meta-analysis. Stroke. 2014;45(6):1778–83.
11. Ormstad H, Eilertsen G. A biopsychosocial model of fatigue and depression following stroke. Medical Hypotheses. 2015;85(6):835–41.
12. Nadarajah M, Goh HT. Post-stroke fatigue: a review on prevalence, correlates, measurement, and management. Top Stroke Rehabil. 2015;22(3):208–20.
13. Cumming TB, Packer M, Kramer SF, English C. The prevalence of fatigue after stroke: A systematic review and meta-analysis. Int J Stroke. 2016;11(9):968–77.
14. Galligan NG, Hevey D, Coen RF, Harbison JA. Clarifying the associations between anxiety, depression and fatigue following stroke. J Health Psychol. 2015;21(12):2863–71.
15. Mead G, Lynch J, Greig C, Young A, Lewis S, Sharpe M. Evaluation of fatigue scales in stroke patients. Stroke. 2007;38(7):2090–5.
16. Kutlubaev MA, Mead GE, Lerdal A. Fatigue after stroke – perspectives and future directions. Int J Stroke. 2015;10(3):280–1.
17. Kirkevold M, Bragstad LK, Bronken BA, Kvigne K, Martinsen R, Gabrielsen EH, et al. Promoting psychosocial well-being following stroke: study protocol for a randomized, controlled trial. BMC Psychol. 2018;3(1):12.
18. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–53.
19. Watkins C, Lightbody C, Sutton C, Holcroft L, Jack C, Dickinson H, et al. Evaluation of a single-item screening tool for depression after stroke: a cohort study. Clin Rehabil. 2007;21(9):846–52.
20. Adams Jr HP, Davis PH, Leira EC, Chang K-C, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke. Neurology. 1999;53(1):126–31.
21. Martinsen R, Kirkevold M, Sveen U. Younger stroke survivors' experiences of family life in a long-term perspective: a narrative hermeneutic phenomenological study. Nurs Res Pract. 2012;2012(Article ID 948791):11.
22. Schepers VP, Visser-Meily AM, Ketelaar M, Lindeman E. Poststroke fatigue: course and its relation to personal and stroke-related factors. Arch Phys Med Rehabil. 2006;87(2):184–8.
23. Hubacher M, Calabrese P, Bassetti C, Carota A, Stöcklin M, Penner I-K. Assessment of post-stroke fatigue: the fatigue scale for motor and cognitive functions. Eur Neurol. 2012;67(6):377–84.
24. Choi-Kwon S, Han SW, Kwon SU, Kim JS. Poststroke fatigue: characteristics and related factors. Cerebrovasc Dis. 2005;19(2):84–90.
25. Lerdal A, Bakken LN, Rasmussen EF, Beiermann C, Ryen S, Pynten S, et al. Physical impairment, depressive symptoms and pre-stroke fatigue are related to fatigue in the acute phase after stroke. Disabil Rehabil. 2011;33(4):334–42.
26. Kim JS. Post-stroke mood and emotional disturbances: pharmacological therapy based on mechanisms. J Stroke. 2016;18(3):244–55.
27. Kouwenhoven SE, Kirkevold M, Engedal K, Kim HS. Depression in acute stroke: prevalence, dominant symptoms and associated factors. A systematic literature review. Disabil Rehabil. 2011;33(7):539–56.
28. Lerdal A, Gay C, Lee K. Curvilinear relationship between age and post-stroke fatigue among patients in the acute phase following first-ever stroke. Int J Phys Med Rehabil. 2013;1(5).
29. Fjærtoft H, Indredavik B, Mørch B, Phan A, Skogseth-Stephani R, Varmdal R. Årsrapport 2016 – med plan for forbedringstiltak. 2017; St. Olavs hospital HF: Nasjonalt sekretariat for Norsk hjerneslagregister, Seksjon for medisinske kvalitetsregistre, Norsk Hjerneslagregister. Available at: https://stolav.no/Medisinskekvalitetsregistre/Norsk-hjerneslagregister/%C3%85rsrapport2016-Norsk-hjerneslagregister.pdf(downloaded 13.10.2018).










Comments